A the nature of the constituent to be separated ie whether it is polar or non-polar. First is the solvent.
Method 5 Separating Funnel What Is A Separating
Vacuum filtration and TLC.

. The solvent is called the mobile phase or eluant. Filtration of the solution leaves sand the filter paper. A Use of suitable solvent.
The liquid in which the solid substance is mixed or gets dissolved is known as a solvent. The choice of solvent or a mixture of solvents used in TLC is solely guided by two important factors. A mixture of unknown amino acids can be separated and identified by means of paper chromatography.
So if you dissolved in 30 mL of DCM youd extract it 3X 10 mL 10 HCl. Remove the stopper and collect the aqueous layer in the 125 mL Erlenmeyer flask labeled hydroxide. This method is best for separating a liquid from a solution.
Weak solvent portion x weak solvent strength a. Keep in mind that phenyl groups are considered fairly nonpolar. If the solvent is of too low a polarity the components will not move enough and again separation will not occur Rfs will be too small.
Solvents for chromatography Less Eluting Strength less polar solvents. Continued on other side. The TLC utilizes chloroform as the mobile phase so that the solutions can be separated by their various polarities.
A mixture of two solids cab be separated by one of the following methods. Click to see full answer. TLC is used to determine if certain compounds in the mixture have been fully removed.
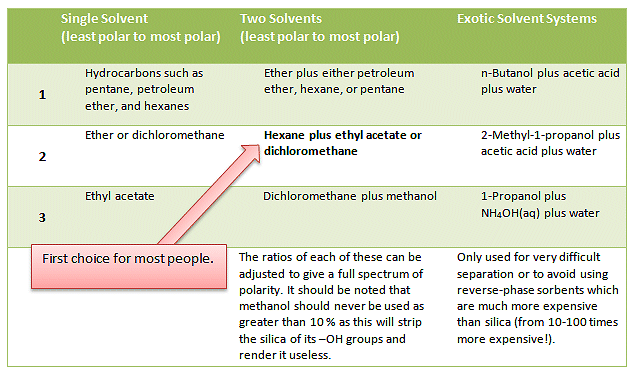
Once you have the right solvent system pack an appropriate silica gel column right column diameter and length and carefully choosing the eluant separate the. Strong solvent portion x strong solvent strength b Equation 1. 35 Votes Filtrationis used to separatea solid from a liquid in which it is suspended.
And b the nature of the process involved ie whether it is a case of adsorption or partition chromatography. For example a mixture of one part acetone and two parts toluene will have a solubility parameter of 187 ⅓ 197 ⅔ 183 about the same as that of chloroform. First if not already the three-component solution should be mixed with an organic solvent like dichloromethane DCM.
Filtrationis also used to separatea substance from a mixturebecause one is insoluble in the solvent and the other is soluble. The separation is due to particle size. The solubility parameter of a solvent mixture can be determined by averaging the Hildebrand value of a solvent of the individual components by volume see eg.
Qualitative separation can be. A b total solvent strength. Well it is simply summing the product of the amount of each solvent and its strength Equation 1.
The process involves heating the solution until the solvent evaporates turns into a gas leaving behind the solid residue. The compounds to be separated are benzophenone biphenyl and benzhydrol also know as diphenylmethanol. These solute and solvents can be separated in their pure.
A mixture of sugar and sand can be separated by adding water as the solvent which dissolve sugar but not sand. Gently shake the separatory funnel to allow intimate mixing of the solutions and effect extraction of the compound from the organic mixture. Separation of Mixture of Solid and Liquid.
Rf distance traveled by componentdistance traveled by solvent Know how to set up a chromatography experiment-Obtain 3 250 mL beakers and label them water alcohol and table salt and cover each with a petri dish-Obtain 3 chromo papers and draw a pencil line 1cm from the bottom of each-Label the 7 dyes every 1cm with pencil starting 15cm from the left edge of the. GBromocresol green SSudan III Rmethyl red Mmixture correlation. Whereas the dissolving solid constituent is known as solute.
In practice different solvents or mixtures of solvents are tried until a good separation is observed. In the first case the chosen extractive solvent should have a higher solubity for the extract than the parent solvent does and it should have a much lower solubility for anything else that might be there. The method which is used for this purpose depends upon the nature of the components present in the mixture.
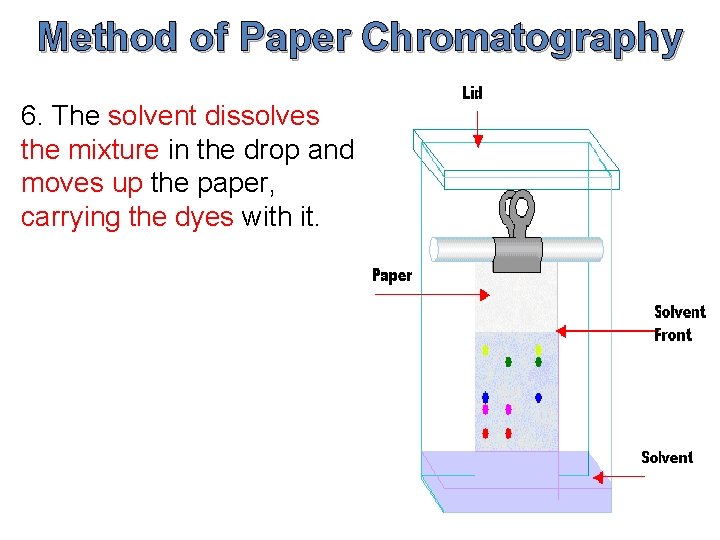
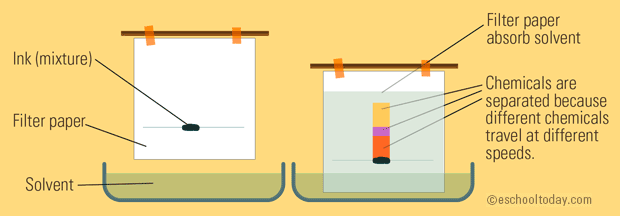
The solvent moves up a piece of filter paper by capillary action. First number is distanced traveled by spot second number is Rf value. The filter paper which contains a thin film of water trapped on it forms the stationary phase.
Extract with 10 HCl. 485659 Views. Evaporation is great for separating a mixture solution of a soluble solid and a solvent.
Mixtures of solvents are employed to achieve optimum separation by TLC. Complications will come if they form azeotrope. A mixture of solid and liquid can be found in a homogeneous solution or heterogeneous mixture.
The structures of these three aromatic compounds are shown in Figure 1. How To Separate Solutions Mixtures Emulsions Chemical Tests Chemistry FuseSchoolLearn the basics about separating solutions mixtures and emulsions. Quantitative separation of a solvent mixture can be carried out by fractional distillation.
When using solvent mixtures it should be kept in mind that addition of only a minor amount of a polar solvent can result in a large increase in the eluting power of the mixture. CLICK HERE to see an illustration of how it works. Third It is better to use separate X ml portions of the extracting solvent 10 times than 10X mls of the solvent once.
Clamp the separatory funnel to a retort stand and allow the mixture to separate into two layers. It has been observed that the rate of migration of a substance on a given. Move along with the solvent and no separation will be observed Rfs will be too large.
Usually you extract 3X with the total volume being roughly equal to what the mixture is dissolved in. The best solvent system to use to separate our three component mixture. As previously mentioned two techniques are vital to solvent extraction.
Tips And Tricks For The Lab Column Choices Education Chemistryviews
Explain The Mixture Separation Techniques Example

Wizer Me Word Problem Worksheets Blends Worksheets Science Worksheets

Mixtures Solutions Hands On Edible Science Activities For The Stem Classroom Vocabulary Word Walls Science Activities Physical Science Lessons

Mixtures And Solutions Chemistry For Kids Solute Solvent Solution Matter Science School Science Experiments Chemistry Education

1 4 Laboratory Techniques For Separation Of Mixtures Chem 1114 Introduction To Chemistry

Principles Of Chromatography Stationary Phase Article Khan Academy

Cambridge Cie Igcse Chemistry Contents Topic 2 Experimental Chemistry Thin Layer Chromatography Chemistry Organic Chemistry

0 Comments